Case Study: How Intricon Resolved Supply Chain Issues and Accelerated Time to Market
From 100% failure to 100% passing, how Intricon resolved tooling and supply chain issues and accelerated time to market.
The Project
An innovative medtech company was unable to bring its revolutionary product to market when its supplier failed to produce components to specification. Frustrated with an unresponsive vendor and costly delays that threatened to derail the project, the company hired Intricon to rapidly resolve product issues, overcome supply chain challenges, and validate component manufacturing for FDA submission.
The Challenge
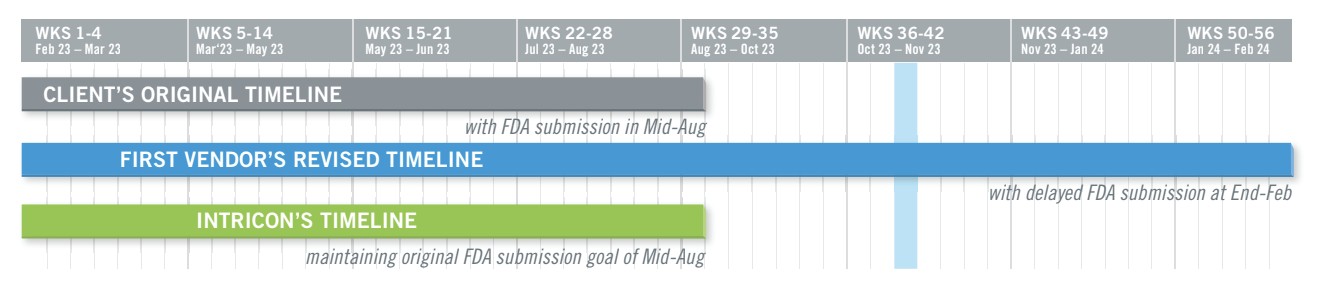
The Intricon team identified design deficiencies in five mold tools that caused poor venting, inadequate cooling, and incorrect steel geometry, yielding unacceptable plastic parts that failed strength testing and exhibited short shots, leak failures, and aesthetic blemishes. Numerous cavities failed acceptance criteria, reducing production capacity. The project demanded rapid resolution as the FDA submission timeline did not allow for corrections, putting the project at risk.
The Solution
Intricon’s in-house injection molding and tooling experts addressed the mold deficiencies for all five tools concurrently, simultaneously establishing sampling and validation plans. The team rapidly resolved a weak part design – exceeding expectations with a twofold improvement in performance – and automated manufacturing with robotics-driven molding, inspection, and parts-handling that eliminated distortion and human error, increased efficiency and yields, and reduced costs. Intricon also leveraged supplier relationships to source alternative materials and packaging options to avoid additional delays.
The previous suppliers had submitted parts that had a 100% test failure rate, but Intricon’s parts had a 100% pass rate. The team also resolved the failing cavitation to achieve 100% acceptance on all leak-tested components.
By working with the customer, Intricon resolved all tooling and molding issues, improved product quality, and enabled the company to achieve their original FDA submission deadline. The customer credits Intricon with accelerating its timeline by at least six months, stating, “As is the norm, Intricon delivers on time or sooner, in many cases pushing us for approvals. Without the expertise and guidance of Intricon, we would not be where we are today.”