Case Study: A Major Surgical Device Company Leverages Intricon to Save a Failed Design and Deliver Solution in Just 2 Days
The Project
A major medical device company faced costly delays when its innovative surgical device failed design validation (DV). The failure threatened to derail the project via missed deadlines, inflated costs, and broken business promises. Suspecting a faulty electromagnetic (EM) sensor, the company turned to Intricon to salvage the project through rapid redesign.
The Challenge
The Intricon team confirmed the client’s suspicion that the EM sensor was susceptible to magnetic interference. They determined that a solution required more than the catalog offerings provided by most suppliers. Rather, the EM sensor needed a total redesign with customization for tracking and performance optimization. Intricon’s in-house EM sensor design and microcoil manufacturing experts rapidly began to customize a component solution so the customer’s device work could resume quickly.
The Solution
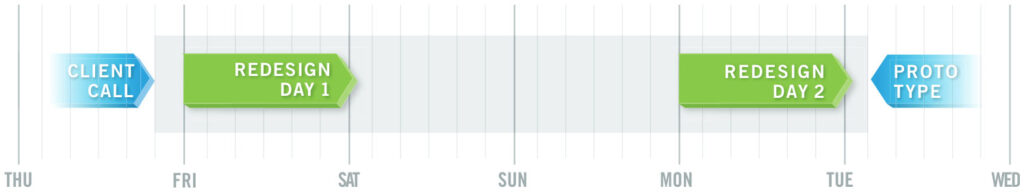
The client called on Thursday evening, and Intricon delivered a first prototype of a newly-designed component that substantially met the challenges—in just two working days. The team leveraged its navigation coil design expertise, performance characterization abilities, and wind process improvements to redesign the EM sensor with a custom form factor that mitigated magnetic interference. Applying its Design for Manufacturing (DFM) approach, Intricon selected materials and developed processes that cut costs in half, in addition to meeting challenging constraints and quality requirements. The client was able to quickly replan design validation and recognized Intricon with its supplier excellence award for rapid response.