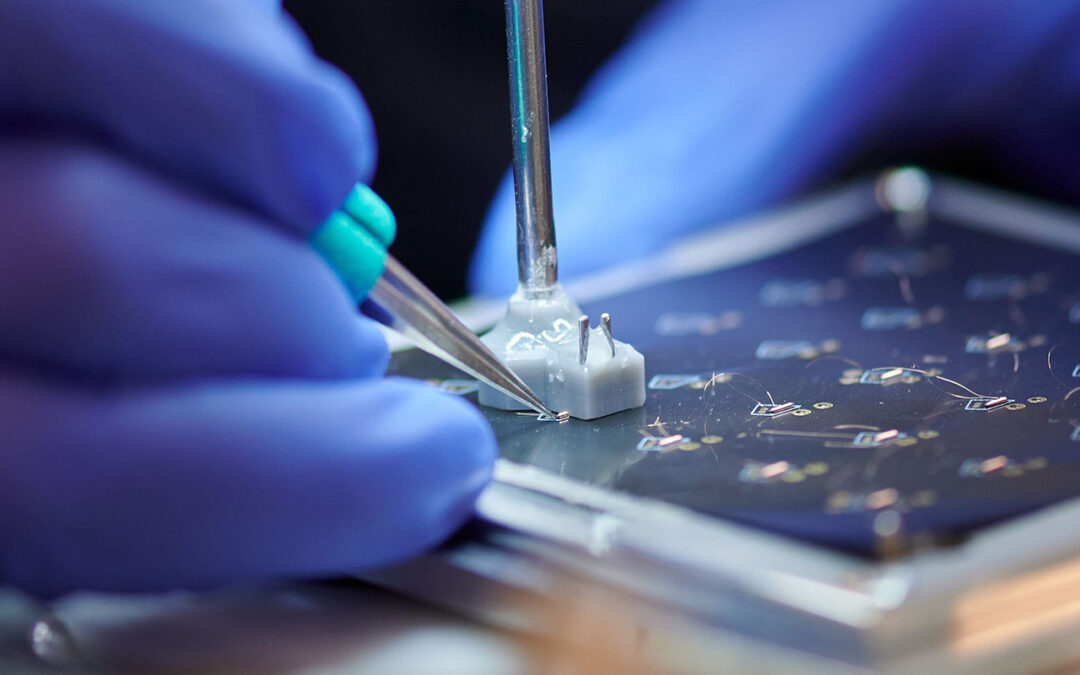
by Zach Harvard | Mar 25, 2025 | Thought Leadership, Uncategorized
Vertical integration is an ongoing trend as medical OEMs shrink their approved supplier lists (ASLs) to consolidate and simplify supply chains. However, the very definition of vertical integration is evolving to address modern challenges. The medical device industry...
by Zach Harvard | Jan 22, 2024 | Thought Leadership, Uncategorized
Intricon’s latest MD&DI article has Intricon’s Darren Gilmer and Trent Birkholz speaking to MD+DI Qmed on component selection considerations. Thoughtful component selection for sensor-driven devices can help avoid pitfalls that can derail...