Biosensors represent a new frontier in medicine, where wearable and implanted devices monitor biological markers to predict, discover, and prevent disease
(see Figure 1). The COVID-19 pandemic accelerated demand for biosensor devices for both consumer and medical use, driving innovation and creating a lucrative market that is projected to grow from $28.5 billion in 2022 to $58 billion by 2032 — with some segments seeing growth rates exceeding 20 percent.1 OEMs and start-ups that seek to bring a biosensor device to the medical market must be strategic in their approach or risk delays and inflated development costs. The design phase is a critical juncture, as decisions made during this stage are often the difference between a commercially successful biosensor device and one destined for significant redesign — or one that might not be viable at all. Here are seven best practices for starting a design process that fosters commercial success for medical biosensors.
1. BEGIN WITH THE END IN MIND
Start by defining end goals:
- Do you want to exit and sell to another business? If so, at what point?
- Do you want to develop and sell the biosensor yourself?
- In which regions will you sell the finished device?
Start-ups and OEMs often have different goals. Start-ups may struggle for cash and have high financial burn rates. They need an exit strategy because they cannot compete on the sales and marketing stage in an industry where the top five players occupy 25–50 percent of market share.2 A company might have the best product, but it won’t succeed if the team can’t identify or create a cost-effective sales channel.
It is important to know the exit plan from the start and consult experts who can recommend design decisions that help strategically time the release of the product to support an ideal exit. For many start-ups, the commercialization timeline needs to be accelerated to align with the exit goal.
OEMs have experience bringing products to market with robust sales and marketing processes. Strong sales and marketing increase demand—which is a good problem. However, when the sales team requests hundreds of products, the development team may only be able to deliver half. Developers get overwhelmed, and development slows. That’s problematic because the cost of time to market is immeasurable if you miss — and you may not recover if a competitor gets established first. It’s critical to evaluate how much the internal team can reasonably accomplish and whether it would be beneficial to creatively partner with sources that can accelerate production—from prototypes to final products.
2. UNDERSTAND END-USER NEEDS
Patient and physician acceptance are two critical yet often overlooked factors to address at the beginning of the design phase. Take the chassis and other form factor considerations, as an example. From the patient perspective, wearable biosensors must be comfortable and inconspicuous. People don’t want biosensor devices that remind them of their fallibility or make them look sick. The most competitive, in-demand products must be easy for the patient to embrace and operate.
Doctors must believe in a device’s reliability if they are going to prescribe it to their patients. They need to know the biosensor will perform and that the patient experience will be positive. Doctors will likely be reluctant to abandon tried-and-true practices, so it’s imperative to anticipate how a product may disrupt their beliefs and workflows and proactively design to preempt any objections.
It’s also important to think beyond your borders to where in the world your biosensor device will be used. U.S. medical device companies dominate the global market— with nearly 70 percent of global share and more than $1 billion in annual revenue. And, U.S. companies tend to have U.S. perspectives, which can be reflected in the product’s design. However, think globally and consider where you’ll be selling and how your device fits culturally and functionally — such as device color, labeling, and ability to function in various climates. This will differentiate the biosensor and earn acceptance in potentially lucrative global markets.
3. PREPARE FOR REGULATORY REQUIREMENTS
Regulatory requirements send many devices to the graveyard. Approximately 25 percent of medical devices submitted for FDA approval are denied every year, underscoring the need to understand and meet regulatory requirements during the design phase.3
In fact, three of the primary constraints in the global biosensor market are tight regulations, complicated reimbursement policies, and the rate at which rules adjust to new technologies.4 A time-to- market analysis conducted by the National Institutes of Health found that the main bottleneck in the transference process is in the clinical trial stage, where failures are often rooted in insufficient design, poor understanding of user requirements, and lack of testing early in the development process.
Know the biosensor device’s classification and research the regulatory guidanceand precedents.5 This can be challenging because, even though rules are documented, real-life requirements go beyond what’s written and include the experience of precedents and related submissions. This is another reason to consult experienced partners who can help design your regulatory strategy and craft your submission to earn approval.
Many companies go too far too fast, thinking they’ll fill in the blanks later. However, it’s worth tapping the brakes to get it right the first time. Taking an extra month or two to meet regulatory requirements is far more cost-effective than re-engineering a biosensor after FDA denial.
4. DESIGN FOR MANUFACTURABILITY
Many great ideas fail because they’re not designed for manufacturability. Failure at this stage:
- Extends production timelines.
- Increases costs.
- Makes it difficult (or impossible) to scale. • Results in poor material selection.
Thus, designing for manufacturability means increased production efficiency, faster production times, lower costs, and improved product reliability. Strategic material selection is a critical factor in cost, reliability, and manufacturability. For example, glue or epoxy may be an expeditious initial choice, but adhesives are difficult to utilize in full-scale manufacturing operations. In many cases, selecting a different material or component — in this case, a mechanical joint vs. glue — maybe a better long-term option. Focusing on the end goal helps inform crucial decisions such as material selection that influence commercial success.
Scalability is likewise crucial. Once you make a reliable product that works, can you do it again? Can you do it 1,000 times? Learn from partners who have done it before to maintain scalability throughout your design process.
5. EVALUATE COMMERCIAL VIABILITY
In addition to patient and physician acceptance, another factor that influences commercial viability is whether the healthcare system can afford the biosensor device. At the design phase, consider:
- Who will pay for the device?
- Can users afford it?
- Will insurers cover it?
- Will Medicare pay for it?
- Is there precedence for a biosensor like yours in the market?
- How will you demonstrate economic superiority?
- Is there an existing reimbursement code for your biosensor?
Although many whiteboard ideas attract development funding, they are not commercially viable. Prior to fundraising, at the design phase, partner with a development and manufacturing company that can provide prototypes of the product that demonstrate commercial viability. Prototypes that have been tested and prove that your product can be cost-effective and efficiently produced to meet demand reduces risk for both the business and its investors.
Establishing intellectual property is also crucial at this stage. Ensure that your technique is unique, protectable, and not in violation of existing patents.
6. ASSESS PROJECT COSTS
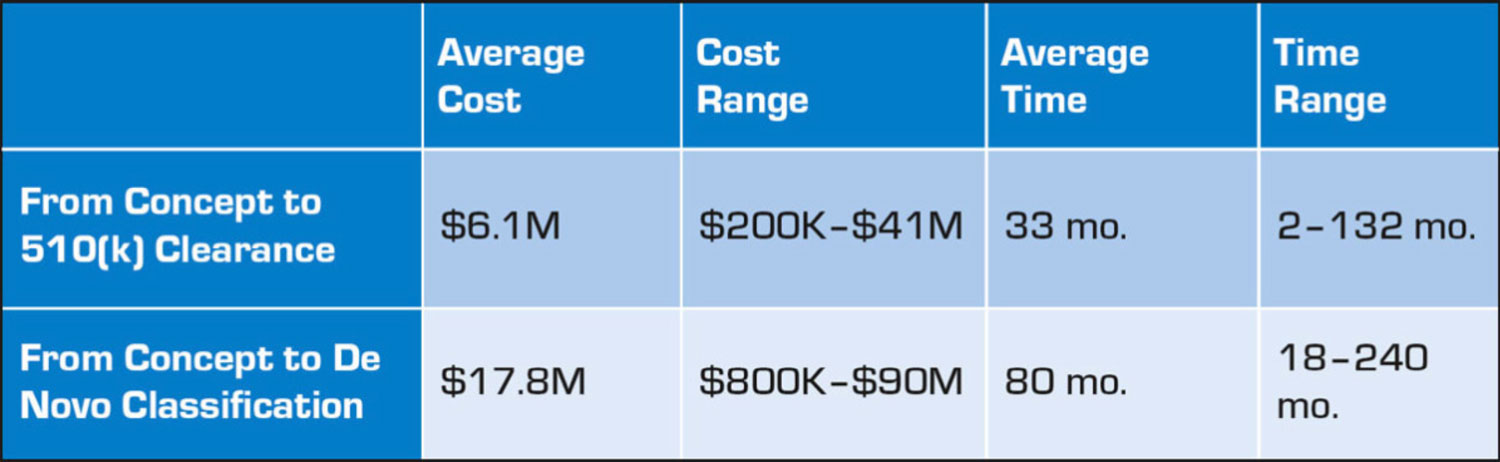
Too often, great ideas fail due to limited funds and resources. Timelines extend and costs naturally increase. With medical devices, including biosensors, costs and timelines vary significantly depending on factors such as complexity and product risk classification. The average cost of developing a medical device ranges from $6.1 million to $17.8 million depending on regulatory classification, with an average timeline to completion ranging from 33 to 80 months (see Table 1).
To maximize the chance of success, carefully project what it will cost to reach your goal and then identify how to leverage funds to reduce risk. Considering the requirements for development and full-scale manufacturing at the design phase helps developers reverse engineer timelines and costs to make them more realistic and achievable.
7. WORK WITH THE RIGHT PEOPLE
Whether you plan to outsource some of the design or do everything in house, working with the right people is paramount. Hire experienced people or partner with companies that have successfully brought medical biosensors to market.
An expert team not only helps a company develop a commercially viable biosensor device, it can also help attract investors. Watch Shark Tank and you’ll note that the management team is one of the most important criteria investors consider. The ability to attract funding is directly tied to the people leading a project. Leaders and design teams should consult:
- Market experts
- Regulatory reimbursement experts.
- Manufacturability experts.
Work with those who have vast experience, whether employees or partners that serve as extensions of your internal team. From the onset of product conception, surround yourself with experienced voices to give your medical biosensor the best chance to succeed.
This article was written by Dave Liebl, Chief Commercial and Technology Officer at Intricon, St. Paul, MN. Intricon partners with medical device companies, providing unique microelectronic expertise—including miniature molding through final assembly—and regulatory guidance, supply chain optimization, and scalable production, exclusively for the medical market.
References
1. “Biosensors Market Size By Type (Wearable, Non- wearable), By Technology (Electrochemical, Optical, Thermal, Piezoelectric), By Medical Application (Blood Glucose Testing, Cholesterol Testing, Blood Gas Analysis, Pregnancy Testing, Drug Discovery, Infectious Disease Testing), By End-use (Point of Care Testing, Home Healthcare Diagnostics, Research Laboratories), Regional Outlook, Industry Analysis Report, Growth Potential, Competitive Market Share & Forecast, 2023–2032,” Global Market Insights.
2. “Biosensors Market by Type, Product (Wearable, Non-wearable), Technology, Application (POC, Home Diagnostics, Research Lab, Environmental Monitoring, Food & Beverages, Biodefense) and Region 2026,” Markets and Markets.
3. Fred Pennic, “Medical Devices Cleared or Approved by FDA in 2022,” HIT Consultant, August 29, 2022.
4. “Biosensors Market Size, Share & Trends Analysis Report By Technology (Thermal, Optical), By Application (Medical, Food Toxicity), By End-user (Home Healthcare Diagnostics, POC Testing), And Segment Forecasts, 2023–2030,” Grand View Research.
5. Rossana E. Madrid, et al., “Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users,” Bioengineering, March 2022.